Table of contents
1.0 Introduction
1.1 Description of occurrence
1.1.1
On 06 July 2013, a unit train carrying petroleum crude oil operated by Montreal, Maine & Atlantic Railway derailed in Lac-Mégantic, Quebec. Numerous tank cars ruptured and a fire ensued. The ambient air temperature at the time of the derailment was reported to be around 21 °C.
1.2 Engineering services requested
1.2.1
A request was received from the Transportation Safety Board of Canada (TSB) Eastern Regional Operations - Rail/Pipeline office to analyze crude oil samples taken from selected tank cars.
2.0 Examination
2.1 Sampling procedure
2.1.1
Crude oil samples were taken from selected tank cars under the direction of a TSB investigator. Table 1 summarizes the sampling details. Samples were collected from the 9 non-derailed tank cars at the end of the occurrence train (MMA-002) that were pulled back to Nantes, Quebec, after the derailment. In addition, samples were taken from 2 tank cars located at Farnham, Quebec, that were part of another unit train operated by Montreal, Maine & Atlantic Railway (MMA-874) that was transporting petroleum crude oil from the same origin as the occurrence train.
2.1.2
No attempt was made to collect samples from the derailed tank cars since all were exposed to the post-derailment fire to some extent. It was considered that this heat exposure would likely have caused volatile components of the crude oil to escape through breaches in the tank and/or during activation of the pressure relief device. Consequently, there was a high probability that any product samples collected from the derailed tank cars would not be representative of the lading prior to the derailment.
2.1.3
Prior to the collection of samples, the vapour space of each tank car was tested using a portable hydrogen sulphide gas detector. No measurable amount of hydrogen sulphide gas was detected.
| Car initial & number | Location collected | Date collected (YY-MM-DD) | Sampling method (see para. 2.1.4) | Quantity collected | Sample identification |
|---|---|---|---|---|---|
| NATX 310533 | Nantes | 13-07-07 | A | 250 mL 250 mL |
NATX310533-A NATX310533-B |
| NATX 310533 | Nantes | 13-08-07 | C | 1000 mL 1000 mL |
NATX310533-C-TOP NATX31533-C-BOT |
| NATX 310595 | Nantes | 13-07-17 | A | 250 mL 250 mL |
NATX310595-A NATX310595-B |
| NATX 310595 | Nantes | 13-08-07 | C | 1000 mL 1000 mL |
NATX310595-C-TOP NATX310595-C-BOT |
| NATX 310406 | Nantes | 13-07-23 | B | 250 mL | NATX310406 |
| NATX 310406 | Nantes | 13-08-08 | C | 1000 mL 1000 mL |
NTAX310406-C-TOP NATX310406-C-BOT |
| WFIX 130629 | Nantes | 13-07-23 | B | 250 mL | WFIX130629 |
| WFIX 130629 | Nantes | 13-08-08 | C | 1000 mL 1000 mL |
WFIX130629-C-TOP WFIX130629-C-BOT |
| PROX 44211 | Nantes | 13-07-23 | B | 250 mL | PROX44211 |
| PROX 44211 | Nantes | 13-08-08 | C | 1000 mL 1000 mL |
PROX44211-C-TOP PROX44211-C-BOT |
| NATX 310425 | Nantes | 13-07-23 | B | 250 mL | NATX310425 |
| NATX 310425 | Nantes | 13-08-08 | C | 1000 mL 1000 mL |
NATX310425-C-TOP NATX310425-C-BOT |
| ACFX 73452 | Nantes | 13-07-23 | B | 250 mL | ACFX73452 |
| ACFX 73452 | Nantes | 13-08-07 | C | 1000 mL 1000 mL |
ACFX73452-C-TOP ACFX73452-C-BOT |
| NATX 310572 | Nantes | 13-07-23 | B | 250 mL | NATX310572 |
| NATX 310572 | Nantes | 13-08-08 | C | 1000 mL 1000 mL |
NATX310572-C-TOP NATX310572-C-BOT |
| NATX 310487 | Nantes | 13-07-23 | B | 250 mL | NATX310487 |
| NATX 310487 | Nantes | 13-08-07 | C | 1000 mL 1000 mL |
NATX310487-C-TOP NATX310487-C-BOT |
| NATX 310487 | Nantes | 13-08-07 | C | 500 mL 500 mL |
NATX310487-D-TOP NATX310487-D-BOT |
| NATX 303425 | Farnham | 13-07-25 | A | 500 mL | NATX303425 |
| PROX 44169 | Farnham | 13-07-25 | B | 500 mL | PROX 44169 |
2.1.4
Three sampling methods (referred to as methods A, B and C in Table 1) were employed in accordance with ASTM D4057. Footnote 1 For method A, a middle sample Footnote 2 was collected using a glass pipette ( -inch diameter, 60-inch long). For method B, an upper sample Footnote 3 was collected using a plastic bailer. Footnote 4 For method C, a peristaltic pump was used to collect lower samples Footnote 5 (identified by the suffix BOT in Table 1) and upper samples (identified by the suffix TOP in Table 1), after verifying that no stratification had occurred in the tank car. This was accomplished by collecting a vertical column of liquid representing the liquid in the tank using a COLIWASA in accordance with ASTM D5495. Footnote 6 Footnote 7 Visual inspection of the COLIWASA samples did not reveal any visible stratification.
2.1.5
All samples were transferred immediately from the sampling tool to glass bottles that were hermetically sealed and stored at ambient temperature until testing. Figure 1 shows 2 representative occurrence crude oil samples. The oil was a dark grey, greenish color.
2.1.6
The crude oil samples were sent for testing to 4 external laboratories Core Lab. Footnote 8, Maxxam Analytical Footnote 9, AITF Footnote 10 and Cassen. Footnote 11 The original analytical reports and certificates of analysis provided by the external laboratories are presented in Appendix A.
2.2 Flash point temperature
2.2.1
The flash point temperature is a measure of the tendency of a test specimen to form a flammable mixture with air under controlled laboratory conditions. The flash point is used in shipping and safety regulations to define flammable and combustible materials and to classify them according to their associated hazard. Footnote 12 Footnote 13 The flash point can indicate the possible presence of highly volatile and flammable constituents in a relatively nonvolatile or nonflammable material.
2.2.2
The ASTM D93 test methods cover the determination of the flash point of petroleum products in the temperature range from 40 to 370 °C by a Pensky-Martens closed-cup apparatus. Footnote 14 Values less than 40 °C can be measured using the D93 procedure but the precision Footnote 15 of such values has not been determined.
2.2.3
The ASTM D3828 test methods cover procedures for flash point of petroleum products and biodiesel liquid fuels within the range of -30 to 300 °C, using a small scale closed cup tester. Footnote 16 It should be noted that flash point values are a function of the operational procedures, design and condition of the apparatus used. Consequently, results obtained using different test methods may not provide valid correlations.
2.2.4
Selected crude oil samples were sent to Core Lab., Maxxam Analytical and AITF for determination of the flash point in accordance with ASTM D93 and ASTM D3828. Samples NATX310406, WFIX130629, NATX303425 and PROX44169 were split so that an approximately 65 mL portion was sent to AITF and the remaining portion (about 185 mL) was sent to Maxxam Analytical. The flash point results are summarized in Table 2. All of the samples gave corrected flash points that were significantly less than 23 °C. Footnote 17 Note that as mentioned previously, the different cut-off points reported by the 3 laboratories reflect the differences in apparatus and method used.
| Sample identification | Laboratory | Test method | Corrected flash point ( °C)Note 1 |
|---|---|---|---|
| NATX310533-A | Core Lab. | ASTM D93 | <-5 |
| NATX310533-B | Maxxam Analytical | ASTM D93 | <-35 |
| NATX310595-A | Core Lab. | ASTM D93 | <-5 |
| NATX310595-B | Maxxam Analytical | ASTM D93 | <-35 |
| NATX310406 | Maxxam Analytical | ASTM D93 | <-35 |
| NATX310406 | AITF | ASTM D3828 | <-30 |
| WFIX130629 | Maxxam Analytical | ASTM D93 | <-35 |
| WFIX130629 | AITF | ASTM D3828 | <-30 |
| PROX44211 | Maxxam Analytical | ASTM D93 | <-35 |
| NATX310425 | Maxxam Analytical | ASTM D93 | <-35 |
| ACFX73452 | Maxxam Analytical | ASTM D93 | <-35 |
| NATX310572 | Maxxam Analytical | ASTM D93 | <-35 |
| NATX310487 | Maxxam Analytical | ASTM D93 | <-35 |
| NATX303425 | Maxxam Analytical | ASTM D93 | <-35 |
| NATX303425 | AITF | ASTM D3828 | <-30 |
| PROX 44169 | Maxxam Analytical | ASTM D93 | <-35 |
| PROX 44169 | AITF | ASTM D3828 | <-30 |
Return to footnote Note 1 referrer observed flash point corrected for ambient barometric pressure.
2.3 Boiling point distribution
2.3.1
The ASTM D86 method (atmospheric distillation) is the basic test method for determining the boiling range characteristics of a petroleum product. Footnote 18 In this method, a 100-mL sample is distilled in a laboratory batch distillation apparatus at ambient pressure and under prescribed conditions. In ASTM D86 distillation, the initial boiling point (IBP) is the corrected temperature reading at the instant the first drop of condensate falls from the lower end of the condenser tube.
2.3.2
The ASTM D7169 method covers the determination of the boiling point distribution and cut point intervals of crude oils and residues using high temperature gas chromatography. Footnote 19 A gas chromatography apparatus is used to obtain a chromatogram of the sample (a plot of carbon signal versus retention time) and the boiling point distribution is calculated from this chromatogram after making appropriate corrections. The IBP is determined as the temperature corresponding to an accumulated 0.5% of eluted sample Footnote 20 after correcting for sample recovery.
2.3.3
The IBP and boiling point distribution of selected crude oil samples were determined by Core Lab., Maxxam Analytical and AITF in accordance with ASTM D86 and ASTM D7169. Table 3 summarizes the IBP results obtained on the crude oil samples. All of the samples tested using the ASTM D86 method gave IBPs ranging from 43.9 to 50.0 °C. The ASTM D86 IBP results obtained by Core Lab. were in good agreement with those obtained by Maxxam Analytical (the difference was 2.0 °C for sample NATX310533 and 4.5 °C for sample NATX310595).
2.3.4
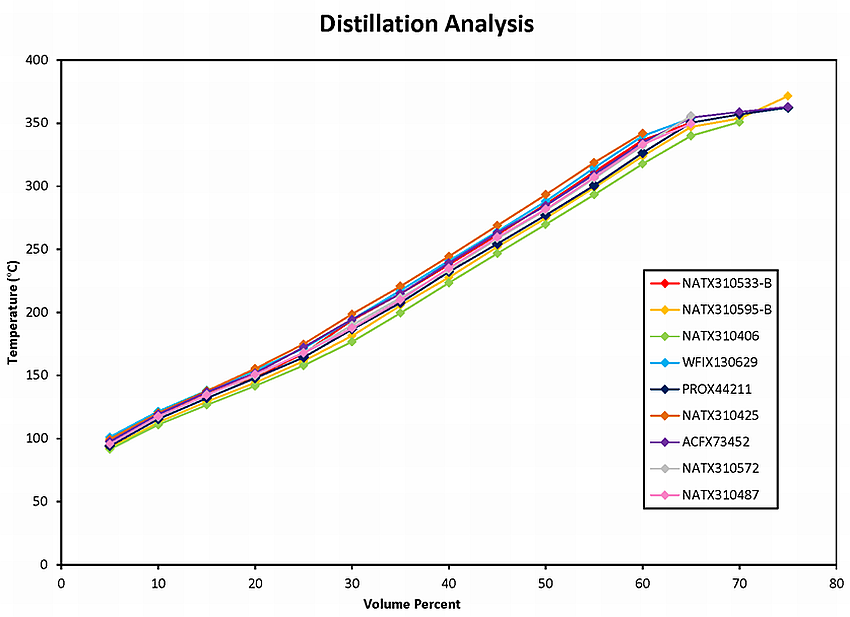
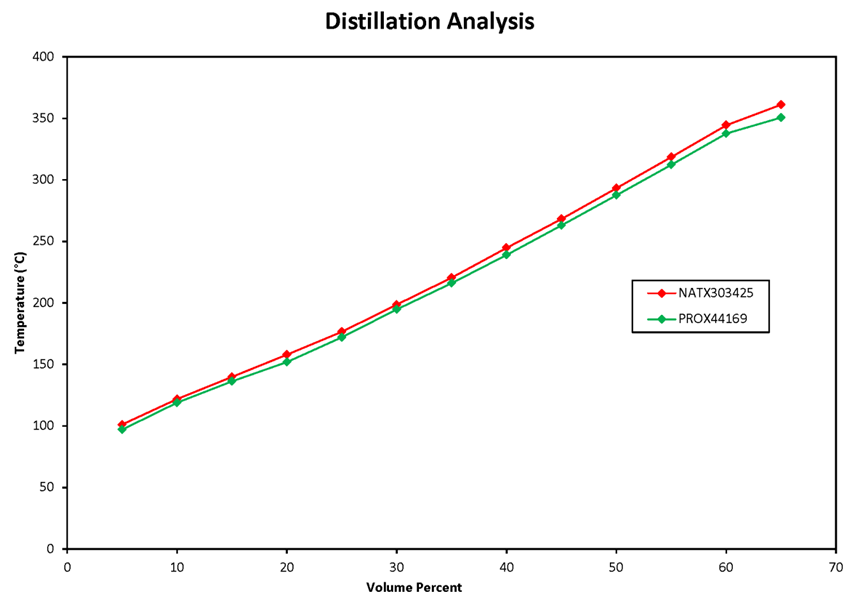
Table 4 summarizes the atmospheric distillation results obtained by Maxxam Analytical for the crude oil samples. The atmospheric distillation analysis is also presented as plots of temperature versus volume percent for the 9 samples collected from the occurrence train (Figure 2) and for the 2 samples collected from the comparison unit train in Farnham (Figure 3). All 11 samples gave very similar boiling point distributions.
2.3.5
There was some concern that the tank cars lading might have been exposed to heat before the tank cars were pulled back to Nantes, thereby affecting the validity of test results. However, no sign of fire damage such as discolored or burned paint was noted on the tail end tank cars. In addition, no unusual variations were noted in the results obtained from the tail end tank car samples. These samples gave very similar results to those obtained from the comparison unit train, which was not exposed to fire (compare Figure 2 and Figure 3).
2.3.6
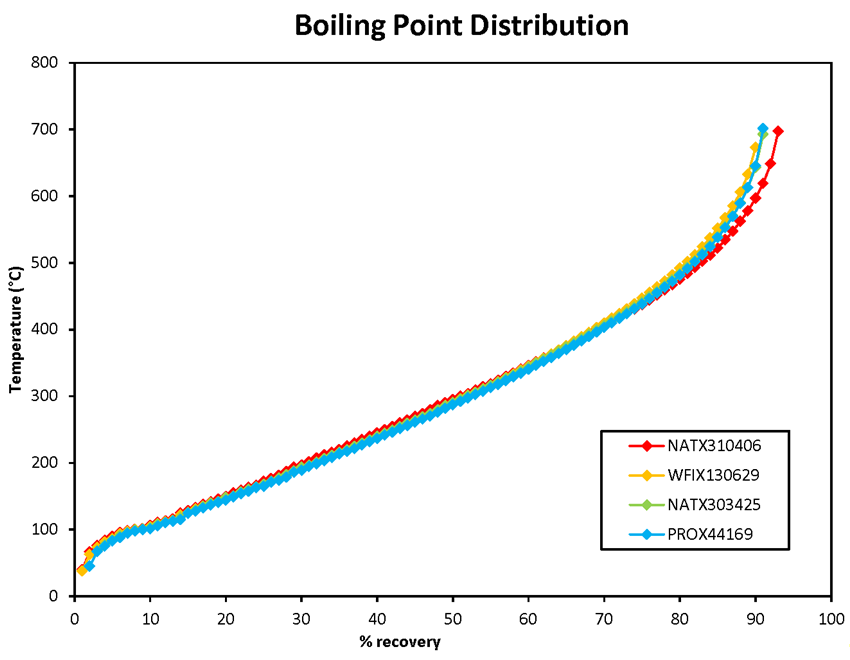
The ASTM D7169 IBP results obtained for the NATX310406, WFIX130629, NATX303425 and PROX44169 samples were at least 10 °C lower than those obtained using the ASTM D86 method (Table 3). Figure 4 displays the boiling point distributions obtained using the ASTM D7169 method. The 4 samples tested using this method gave similar results. It was noted that the ASTM D7169 method gives slightly higher percent recovered values than the ASTM D86 method in the low boiling point portion of the plot which corresponds to the lighter hydrocarbons (compare Figure 2 and Figure 4). As mentioned previously, the 2 methods have a different definition of IBP and use completely different equipment. Consequently, the temperature ranges covered and the precision are different. This likely explains the different results obtained for the light end portion of the samples.
| Sample identification | Laboratory | Test method | Initial boiling point ( C)Note 1 |
|---|---|---|---|
| NATX310533-A | Core Lab. | ASTM D86 | 48.0 |
| NATX310533-B | Maxxam Analytical | ASTM D86 | 46.0 |
| NATX310595-A | Core Lab. | ASTM D86 | 50.0 |
| NATX310595-B | Maxxam Analytical | ASTM D86 | 45.5 |
| NATX310406 | Maxxam Analytical | ASTM D86 | 46.2 |
| NATX310406 | AITF | ASTM D7169 | <36.1 |
| WFIX130629 | Maxxam Analytical | ASTM D86 | 46.7 |
| WFIX130629 | AITF | ASTM D7169 | <36.1 |
| PROX44211 | Maxxam Analytical | ASTM D86 | 48.5 |
| NATX310425 | Maxxam Analytical | ASTM D86 | 44.7 |
| ACFX73452 | Maxxam Analytical | ASTM D86 | 48.5 |
| NATX310572 | Maxxam Analytical | ASTM D86 | 43.9 |
| NATX310487 | Maxxam Analytical | ASTM D86 | 46.3 |
| NATX303425 | Maxxam Analytical | ASTM D86 | 46.2 |
| NATX303425 | AITF | ASTM D7169 | <36.1 |
| PROX44169 | Maxxam Analytical | ASTM D86 | 46.3 |
| PROX44169 | AITF | ASTM D7169 | <36.1 |
Return to footnote Note 1 referrer ASTM D86 results corrected to 101.3 kPa.
| Sample id. | Distillation residue (vol. %) |
Distillation recovery (vol. %) |
Distillation loss (vol. %) |
Distillation naphta (vol. %) |
Distillation kerosene (vol. %) |
|---|---|---|---|---|---|
| NATX310533-B | 32.6 | 66.4 | 1.0 | 32.4 | 15.2 |
| NATX310595-B | 23.8 | 75.2 | 1.0 | 34.7 | 15.2 |
| NATX310406 | 26.2 | 72.8 | 1.0 | 35.9 | 15.0 |
| WFIX130629 | 32.9 | 66.1 | 1.0 | 32.1 | 15.0 |
| PROX44211 | 23.1 | 75.9 | 1.0 | 34.1 | 15.2 |
| NATX310425 | 34.3 | 64.7 | 1.0 | 31.2 | 14.8 |
| ACFX73452 | 19.7 | 79.3 | 1.0 | 32.4 | 15.2 |
| NATX310572 | 30.3 | 68.7 | 1.0 | 33.3 | 15.2 |
| NATX310487 | 31.7 | 67.3 | 1.0 | 33.7 | 14.5 |
| NATX303425 | 33.8 | 65.2 | 1.0 | 31.3 | 14.9 |
| PROX44169 | 32.8 | 66.2 | 1.0 | 32.2 | 15.1 |
2.4 Density analysis
2.4.1
The ASTM D5002 method covers the determination of the density and relative density of crude oils that can be handled as liquids at temperatures between 15 and 35 °C. Footnote 21 The density is defined as the mass per unit volume at a specified temperature. The relative density is the ratio of the density of a material to the density of water at a stated temperature. The API Gravity is a special function of the relative density at 15.56 °C (60 °F) and is calculated as follows: Footnote 22
2.4.2
Four representative crude oil samples were sent to Maxxam Analytical for density analysis. Samples were selected from the tank cars that had given the lowest and highest IBP results (NATX 310572 and PROX 44211 - refer to Table 3). Lower and upper samples were tested for each to verify if any density gradient was present.
2.4.3
The results indicate that the samples collected from tank cars NATX 310572 and PROX 44211 had similar density properties (Table 5). There was no significant difference between the upper and lower samples. This is consistent with the absence of stratification in the tank cars that was visually determined when samples were collected (see paragraph 2.1.4).
| Sample identification | Density at 15 °C (kg/m3) | Relative density at 15 °C | API Gravity |
|---|---|---|---|
| NATX310572-C-TOP | 815.9 | 0.8166 | 41.8 |
| NATX310572-C-BOT | 816.5 | 0.8172 | 41.7 |
| PROX44211-C-TOP | 821.9 | 0.8226 | 40.5 |
| PROX44211-C-BOT | 821.8 | 0.8225 | 40.5 |
2.5 Reid vapour pressure
2.5.1
Vapour pressure of crude oils is an important physical property that affects general handling and refinery practices. It is also used as an indirect measure of the evaporation rate of volatile petroleum products. The ASTM D323 test method is used to determine the vapour pressure at 37.8 °C (100 °F) of petroleum products and crude oils with IBPs above 0 °C (32 °F). Footnote 23
2.5.2
The Reid vapour pressure of the 4 crude oil samples sent to Maxxam Analytical was determined in accordance with ASTM D323 Procedure A. The results indicate that samples collected from tank cars NATX 310572 and PROX 44211 had similar Reid vapour pressures ranging from 62.3 to 66.1 kPa (Table 6). There was no significant difference between the upper and lower samples.
| Sample identification | Reid vapour pressure (kPa) | Total sulphur (mass %) |
|---|---|---|
| NATX310572-C-TOP | 66.1 | 0.096 |
| NATX310572-C-BOT | 64.3 | 0.096 |
| PROX44211-C-TOP | 62.3 | 0.117 |
| PROX44211-C-BOT | 62.4 | 0.117 |
2.6 Sulphur content
2.6.1
The sulphur content of crude oils affects their corrosiveness and toxicity. The ASTM D4294 test method covers the measurement of sulphur in hydrocarbons in the concentration range 0.0150 to 5.00 mass % sulphur. Footnote 24 The total sulphur content of the 4 samples sent to Maxxam Analytical was determined in accordance with ASTM D4294. The results indicate that the crude oil samples contained 0.096 to 0.117 mass % sulphur (Table 6). There was no difference between the upper and lower samples.
2.7 Fluidity pour point and viscosity
2.7.1
Pour point and viscosity determinations are used mainly to determine the handling characteristics of crude oils at low temperatures. The fluidity properties are also indicative of the crude oil composition. For example, crude oils with a greater concentration of paraffinic compounds generally have a higher viscosity than crude oils having higher concentrations of aromatic and naphthenic compounds. Footnote 25
2.7.2
The ASTM D5853 method covers the determination of the pour point of crude oils. Footnote 26 A sample is cooled at a specified rate and examined at intervals of 3 °C for flow characteristics. The pour point is the lowest temperature at which movement of the specimen is observed. Table 7 presents the pour point results obtained on the 4 samples sent to Maxxam Analytical. All of the samples gave pour points below -65 °C.| Sample identification | Pour point ( C) | Kinematic viscosity (mm2/s) Note 1 | |||
|---|---|---|---|---|---|
| Viscosity at 10 °C |
Viscosity at 20 °C |
Viscosity at 30 °C |
Viscosity at 40 °C |
||
| NATX310572-C-TOP | <-65 | 3.639 | 2.882 | 2.295 | 1.910 |
| NATX310572-C-BOT | <-65 | 3.720 | 2.982 | 2.467 | 2.080 |
| PROX44211-C-TOP | <-65 | 4.100 | 3.259 | 2.665 | 2.230 |
| PROX44211-C-BOT | <-65 | 4.078 | 3.220 | 2.548 | 2.205 |
Return to footnote Note 1 referrer 1 mm2/s = 1 centistoke (cSt).
2.7.3
The ASTM D7042 test method specifies a procedure for concurrent measurement of the dynamic viscosity and density of liquid petroleum products and crude oils. Footnote 27 The dynamic viscosity is a measure of the resistance to flow of a liquid under external shear forces. The kinematic viscosity is a measure of the resistance to flow of the liquid under gravity. The kinematic viscosity is obtained by dividing the dynamic viscosity by the density obtained at the same temperature.
2.7.4
The kinematic viscosity of the 4 samples sent to Maxxam Analytical was determined using a Stabinger viscometer in accordance with ASTM D7042. The samples were tested at 20 °C, 30 °C and 40 °C and these results were used to extrapolate the viscosity at 10 °C. The results are summarized in Table 7. Slightly higher values were obtained at each temperature for the samples collected from the PROX 44211 tank car than for those collected from the NATX 310572 tank car. In the case of the NATX 310572 samples, the lower sample (NATX310572-C-BOT) gave slightly higher results at each temperature than the upper sample (NATX310572-C-TOP). This trend was reversed for the PROX 44211 samples.
2.8 Heat of combustion
2.8.1
The ASTM D240 test method Footnote 28 covers the determination of the heat of combustion of liquid hydrocarbon fuels ranging in volatility from light distillates to that of residual fuels. The heat of combustion is a measure of the energy available from a given fuel. The gross heat of combustion is defined in ASTM D240 as the quantity of energy released when a unit mass of fuel is burned in a constant volume enclosure, with the products being gaseous, other than water that is condensed to the liquid state.
2.8.2
Table 8 summarizes the gross heat of combustion results obtained on the 4 crude oil samples sent to Maxxam Analytical. Similar results were obtained for the 4 samples, ranging from 18,445 to 19,416 Btu/lb Footnote 29 (42.905 to 45.160 MJ/kg). The upper samples (NATX310572-C-TOP and PROX44211-C-TOP) gave slightly higher values than the corresponding lower samples (NATX310572‑C‑BOT and PROX44211-C-BOT).
| Sample identification | Gross heat of combustion | |
|---|---|---|
| (Btu/lb) | (MJ/kg) Note 1 | |
| NATX310572-C-TOP | 19,247 | 44.770 |
| NATX310572-C-BOT | 18,445 | 42.905 |
| PROX44211-C-TOP | 19,416 | 45.160 |
| PROX44211-C-BOT | 19,164 | 44.575 |
Return to footnote Note 1 referrer 1 Btu/lb = 0.002326 MJ/kg.
2.9 BTEX compounds
2.9.1
BTEX is the acronym used for a group of volatile aromatic compounds (VOCs): benzene, toluene, ethylbenzene and the xylene isomers. Footnote 30 Footnote 31 The BTEX compounds occur naturally as constituents of crude oil. They are the most soluble and mobile fraction of crude oil and consequently, readily enter soil and ground water during accidental spills. These substances have toxic effects and are subject to occupational exposure limits. BTEX are classified as priority pollutants regulated by Environment Canada and the U.S. Environmental Protection Agency.
2.9.2
Aliquots (20 mL in volume) were taken from 4 selected crude oil samples and sent to the Cassen laboratory for BTEX analysis using a gas chromatography mass spectrometry (GC/MS) method. Footnote 32 The results are summarized in Table 9. The benzene content measured in the 4 samples ranged from 1470 to 1850 ppm Footnote 33 (0.147 to 0.185%). Overall, the concentrations obtained for the BTEX compounds ranged from a lowest result of 768 ppm (0.0768%) for toluene to a highest result of 3500 ppm (0.35%) for m/p-xylene. Footnote 34
| Analyte | CAS number Footnote 35 | Analytical results (ppm) | |||
|---|---|---|---|---|---|
| NATX310572-C-TOP | NATX310533-C-TOP | NATX310595-C-TOP | ACFX73452-C-TOP | ||
| Benzene | 71-43-2 | 1850 | 1720 | 1800 | 1470 |
| Toluene | 108-88-3 | 3170 | 2870 | 2920 | 2770 |
| Ethylbenzene | 100-41-4 | 850 | 768 | 789 | 852 |
| m/p-Xylene | 106-42-3 | 3500 | 3300 | 3310 | 2890 |
| o-Xylene | 95-47-6 | 1660 | 1560 | 1620 | 1500 |
3.0 Discussion
3.1 Classification of the occurrence crude oil
3.1.1
According to the Transportation of Dangerous Goods (TDG) regulations Footnote 36 and the U.S. Code of Federal Regulations Title 49 Footnote 37, liquids or liquids containing solids in solution or suspension are included in Class 3, Flammable Liquids, if they have a flash point less than or equal to 60 °C using the closed-cup test method. Flammable liquids are further classified in one of three packing groups:
- Packing Group I, if they have an initial boiling point of 35 °C or less at an absolute pressure of 101.3 kPa and any flash point;
- Packing Group II, if they have an initial boiling point greater than 35 °C at an absolute pressure of 101.3 kPa and a flash point less than 23 °C; or
- Packing Group III, if the criteria for inclusion in Packing Group I or II are not met.
3.1.2
The flash point results obtained for the subject crude oil samples were all significantly less than 23 °C (Table 2) whereas the IBP results determined using the ASTM D86 method ranged from 43.9 to 50.0 °C (Table 3). Consequently, all of these crude oil samples met the criteria for Class 3, Packing Group II.
3.2 Chemical and physical properties of the occurrence crude oil
3.2.1
The chemical and physical test results obtained on the 9 occurrence crude oil samples show that there was little variation from tank car to tank car. Lower and upper samples gave similar results suggesting there was no significant stratification of the liquid phase within the tank cars.
3.2.2
Petroleum crude oil has been defined as A complex combination of hydrocarbons. It consists predominantly of aliphatic, alicyclic and aromatic hydrocarbons. It may also contain small amounts of nitrogen, oxygen and sulphur compounds. This category encompasses light, medium, and heavy petroleums, as well as the oils extracted from tar sands. Footnote 38 Crude oils are natural products and their chemical and physical properties can vary widely depending upon their origin and extraction method.
3.2.3
Conventional oil, which can range from light to medium in grade, is found in reservoir rocks with sufficient permeability to allow the oil to flow through the rock to a well. The petroleum crude oil on the occurrence train originated from suppliers with producing wells in the Bakken Shale formation region of North Dakota. The Bakken Shale formation is a tight oil reservoir. Tight oil is a type of conventional oil that is found within reservoirs with very low permeability. Most oil produced from low-permeability reservoirs is of the light to medium variety, with a lower viscosity. Advanced production technologies such as horizontal drilling coupled with multi-stage fracturing are required to extract the oil from these tight reservoirs. Footnote 39 The hydraulic fracturing process applies pressure by pumping fluids into the wellbore to open up pathways through which the oil can flow into the wellbore. Water is commonly used as the main constituent of the fracturing process fluid to which small amounts of different additives are added to reduce friction and to prevent corrosion and biofouling. Footnote 40
3.2.4
Table 10 compares the property results obtained for the occurrence crude oil samples with published values for petroleum products ranging from condensate to heavy crude oil. For simplicity, only the upper samples (NATX310572-C-TOP and PROX44211-C-TOP) are shown since similar results were obtained for upper and lower samples. The published values are taken from the 2013 Crude Characteristics Booklet Footnote 41, which is a summary of selected chemical and physical properties of crude oils moved in the Enbridge Pipelines/Enbridge Energy Partners system.
3.2.5
The National Energy Board of Canada (NEB) defines light crude oil as oil having a density equal to, or less than, 875.7 kg/m3. Footnote 42 The density of the occurrence crude oil samples ranged from 815.9 to 821.9 kg/m3, which meets the NEB definition for light crude oil . These density results were similar to the density reported for MST (Manitoba Sweet Tundra), a light crude oil product (Table 10). The vapour pressure and viscosity properties of the occurrence crude oil samples were also similar to those reported for MST. Heavy crude oils Footnote 43 have significantly lower vapour pressure, higher density and much higher viscosity than light crude oils - see for example the WCB product in Table 10.
3.2.6
Condensates are mixtures of light hydrocarbons (with some dissolved hydrocarbon gases such as butane and propane) that remain liquid under modest pressures at ambient temperatures. Condensate products are recovered mainly from gas reservoirs and have significantly lower density and viscosity than other crude oils - see for example the CPM (Pembina Condensate) product in Table 10. Published analyses indicate that CPM contains about 80 vol% total C12- (hydrocarbons with 12 carbon atoms or less). Footnote 44 It is interesting to note that the occurrence crude oil samples and MST product have similar vapour pressure as CPM, suggesting that their volatility is similar to that of this condensate product. Flash points are not reported in the 2013 Crude Characteristics Booklet.
| Source | Product identifier | Total sulphur (mass %) | Reid vapour pressure (kPa) | Density (kg/m3) | Viscosity (cSt) at temperature | |||
|---|---|---|---|---|---|---|---|---|
| 10 °C | 20 °C | 30 °C | 40 °C | |||||
| Occurrence test results | NATX310572-C-TOP | 0.096 | 66.1 | 815.9 | 3.639 | 2.882 | 2.295 | 1.910 |
| PROX44211-C-TOP | 0.117 | 62.3 | 821.9 | 4.100 | 3.259 | 2.665 | 2.230 | |
| 2013 Crude Characteristics Booklet | CPM (Pembina Condensate) | 0.10 | 70.6 | 757.4 | 1.21 | 1.07 | 0.960 | 0.860 |
| MST (Manitoba Sweet Tundra) | 0.41 | 71.0 | 825.3 | 4.44 | 3.50 | 2.83 | 2.36 | |
| WCB (Western Canadian Blend) | 3.04 | 22.0 | 927.5 | 285 | 149 | 85.4 | 53.1 | |
3.2.7
The Environmental Technology Centre (ETC) Oil Properties Database reports the following properties for unleaded gasoline: Footnote 45
- Flash point -30 °C
- Density at 15 °C 750 to 850 kg/m3
- Kinematic viscosity <1 cSt at 38 °C
Comparing these values to the occurrence crude oil results summarized in Table 2, it is apparent that the occurrence crude oil s flash point is similar to that of unleaded gasoline. The density results obtained for the occurrence crude oil samples (see Table 10) are also within the range reported for unleaded gasoline. However, unleaded gasoline has lower viscosity than the occurrence crude oil samples.
3.3 Sulphur content of the occurrence crude oil
3.3.1
The Canadian Center for Energy defines sweet crude oil as oil containing less than 0.5 percent sulphur. Footnote 46 In the present case, sulphur analysis of representative occurrence crude oil samples gave total sulphur results ranging from 0.096 to 0.117 mass %, meeting the Canadian Center for Energy s definition for sweet crude oil. The total sulphur content of the occurrence crude oil is lower than that reported for the MST product and similar to the CPM product (Table 10). In comparison, the WCB product has significantly higher sulphur content, placing it in the sour crude category.
3.3.2
Hydrogen sulphide is a toxic gas that can be present as a dissolved compound in crude oil. It can also be evolved when sulphur compounds in the crude oil decompose during distillation or other heating processes. During an oil spill, the presence of hydrogen sulphide is a safety concern since it is extremely flammable and toxic. Footnote 47 In the present case, CTEH Footnote 48 monitored the derailment site during the TSB field investigation. No detectable levels of hydrogen sulphide were found. This is consistent with the low total sulphur content measured in the occurrence crude oil samples.
3.4 BTEX in the occurrence crude oil
3.4.1
The occurrence crude oil s BTEX content (Table 9) is comparable to typical values reported for crude oils. Footnote 49 Table 11 summarizes some of the exposure limits recommended for BTEX compounds. CTEH reported benzene and other VOC contents well above these exposure limits in portions of the derailment site that were extensively contaminated with the spilled crude oil. Footnote 50 This is consistent with the significant concentrations of benzene and other VOCs measured in the occurrence crude oil samples (Table 9).
| Substance | ACGIH Footnote 52 TLV Footnote 53 (ppm) |
Exposure guideline comments |
|---|---|---|
| Benzene | 2.5 | Short term exposure limit (15 min) Confirmed human carcinogen |
| Toluene | 20 | Time-weighted average (8 h) Not classifiable as human carcinogen |
| Ethylbenzene | 20 | Short term exposure limit (15 min) Possibly carcinogenic to humans |
| Xylene | 100 | Time-weighted average (8 h) Not classifiable as human carcinogen |
3.5 Effect of crude oil properties on the post-derailment spill and fire
3.5.1
Some of the properties that determine crude oil s behaviour and effects during an oil spill incident are: Footnote 54
- the extent to which the oil evaporates, which is related to its vapour pressure;
- the rate at which spilled oil spreads and the extent to which it penetrates the soil, which depends on its viscosity;
- density of the oil, which determines if it is likely to sink or float on water;
- health hazards to on-site personnel from volatile organic compounds and hydrogen sulphide (if present).
3.5.2
Overall, the occurrence crude oil gave low density, low total sulphur, low viscosity, low pour point and low flash point results, generally comparable with other light sweet crude oil products. A high vapour pressure was measured on the occurrence samples, similar to those reported for other light sweet crude oil and condensate products. The IBPs determined by the ASTM D7169 (gas chromatography) method were below 36 °C, corresponding to the normal boiling point for pentane (C5). Footnote 55 This suggests there was some content of lighter hydrocarbons in the samples, consistent with their high vapour pressure results.
3.5.3
The low flash point, low IBP and high vapour pressure results obtained for the occurrence crude oil samples suggest that these samples contained some very light hydrocarbons. Given that the occurrence crude oil samples were taken at atmospheric pressure, this could lead to an underestimation of the volatility of the crude oil as the concentration of light hydrocarbons may have been higher at the time of loading, and later reduced due to evaporation losses.
3.5.4
TSB is unaware of any standard methods intended to sample and to quantify the liquefied and/or dissolved gas content of crude oil in tank cars. Although the ASTM D3700 standard practice covers the equipment and procedures for obtaining representative samples of single-phase liquefied petroleum gas (LPG), Footnote 56 this practice is not intended for non-specification products that contain significant amounts of dissolved gases, free water or other separated phases, such as raw or unprocessed gas/liquids mixtures and related materials. The same equipment could be used for this purpose but additional precautions would be needed to obtain representative samples.
3.5.5
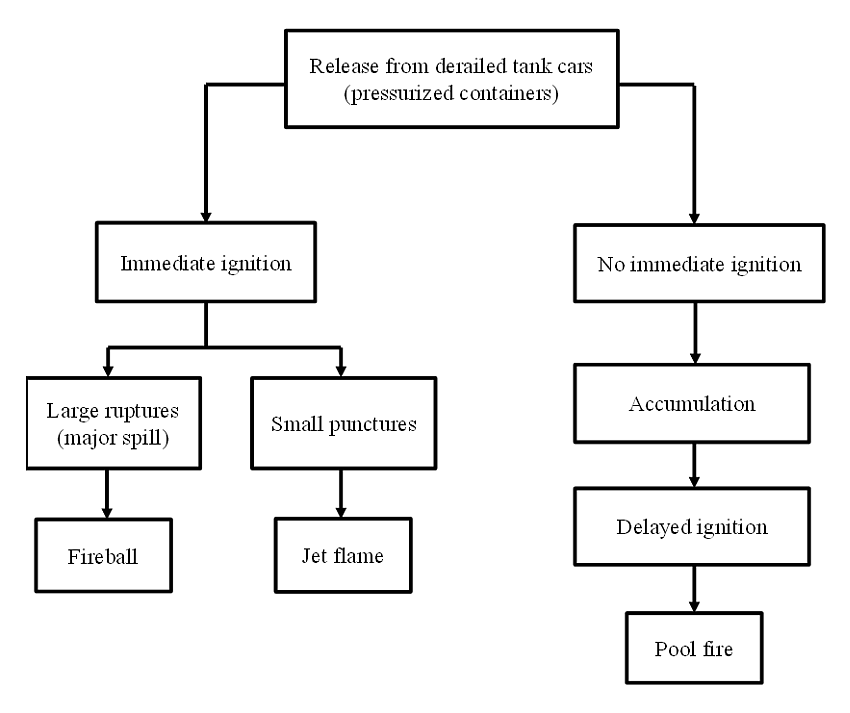
The event tree for the release of crude oil from derailed tank cars can follow 2 pathways depending upon whether the release is accompanied or not by immediate ignition (Figure 5). Ignition is defined as the onset of combustion (flaming) and 3 conditions must be fulfilled for ignition to occur: Footnote 57
- the material must emanate sufficient quantities of vapours or gases;
- the vapours or gases must be mixed with a sufficient quantity of oxidant (oxygen in air);
- the air-vapour mixture must be at a temperature high enough to auto-ignite (self-accelerative oxidation) or a source of ignition (a spark, small flame or other localized source of heat) must be provided.
3.5.6
In the present case, a large number of tank cars sustained large ruptures during the derailment and released their content very rapidly. The spilled crude oil had high vapour pressure and a low flash point (<-35 °C) that was much lower than the temperature at the time of the occurrence (21 °C), indicating it was readily ignitable. Multiple sources of ignition were present at the derailment site such as damaged power lines, derailed equipment, etc. Therefore, all of the conditions required for ignition to occur were present. When the release is a large spill accompanied by immediate ignition (left branch on Figure 5), the result is usually a fireball. The size of this fireball will depend strongly on the amount of flash vaporization and liquid entrainment that occur during the release. Footnote 58 This suggests that more volatile materials (with higher vapour pressure) and high speed derailments (with more energetic impacts and release of lading) will result in larger fireballs. Spilled material that does not ignite immediately (right branch on Figure 5) will spread and accumulate into a pool. The size of this pool will continue to increase until a physical boundary is reached or the material is ignited and burns, resulting in a pool fire.
3.5.7
The viscosity of the occurrence crude oil was similar to that of other light sweet crude oil products; hence it would be expected to have similar spreading characteristics during a spill. The occurrence crude oil s low viscosity was likely contributory to the rapid spread of the spill and flow of crude oil through the town towards the lake. The occurrence crude oil was very volatile, as indicated by its low flash point and high vapour pressure. To summarize, it is considered that the large quantities of spilled crude oil, the rapid rate of release and the oil s high volatility and low viscosity were likely the major contributors to the large fireball and pool fire.
3.5.8
The heat of combustion (also called heating value) is a measure of the total amount of energy that can be released when a fuel is burned to completion. Table 12 compares the gross heat of combustion obtained for the occurrence crude oil samples with values reported in the available literature for other types of fuels. Footnote 59 The results obtained for the occurrence crude oil samples are similar to those reported for crude oil, gasoline and diesel fuels, indicating that all of these fuels will release similar amounts of energy under ideal conditions where fuel is burned to completion. However, it is known that this is never the case in real fires. Even under conditions of unrestricted ventilation (in open air), the combustion products contain compounds that are only partially oxidized such as carbon monoxide, aldehydes, ketones and soot (carbon) particles, indicating that not all of the available energy has been released. Footnote 60
| Product | Heat of combustion (MJ/kg) | Density (kg/m3) | Reference |
|---|---|---|---|
| Occurrence crude oil samples | 42.905 to 45.160 | 815.9 to 821.9 | Table 8 |
| Crude oil | 45.543 | 821.8 | Biomass Energy Data Book |
| Conventional gasoline | 46.536 | 722.8 | Biomass Energy Data Book |
| Conventional diesel | 45.766 | 812.1 | Biomass Energy Data Book |
| Ethanol | 29.847 | 766.2 | Biomass Energy Data Book |
| Liquefied petroleum gas | 50.152 | 493.1 | Biomass Energy Data Book |
3.5.9
The thermal radiation hazards from hydrocarbon pool fires are known to depend on parameters such as the hydrocarbon composition, size and shape of the pool, duration of the fire and the proximity and thermal characteristics of objects exposed to the fire. Footnote 61 Semi-empirical methods are used to estimate the thermal radiation field surrounding a fire. The estimation of the thermal radiation field surrounding the occurrence fire is beyond the scope of the present report. However, temperatures within pool fires have been reported in the available literature. Over a wide range of pool sizes (0.1 to 50 m in diameter), the maximum time-averaged flame temperatures were found to be approximately 900 to 1100 °C, irrespective of the type of fuel. Footnote 62
4.0 Conclusion
4.1
The flash point obtained for the occurrence crude oil samples was significantly less than 23 °C and the IBP determined using the ASTM D86 method ranged from 43.9 to 50.0 °C. Consequently, the crude oil samples clearly met the federal regulatory criteria for being classified as a flammable liquid of Class 3, Packing Group II.
4.2
The occurrence crude oil samples gave low density (815.9 to 821.9 kg/m3), low total sulphur (0.096 to 0.117 mass %), low viscosity (2.882 to 3.259 cSt at 20 °C), low pour point (<-65 °C), low flash point (<-35 °C) and high Reid vapour pressure (62.3 to 66.1kPa) results.
4.3
The occurrence crude oil s properties were consistent with those of a light sweet crude oil, with volatility comparable to that of a condensate or gasoline product.
4.4
There was no indication that the occurrence crude oil s properties had been affected by contamination from fracturing process fluid additives.
4.5
The occurrence crude oil samples were taken at atmospheric pressure. This could lead to an underestimation of the crude oil s volatility due to evaporation loss of very light constituents.
4.6
The large quantities of spilled crude oil, the rapid rate of release, and the oil s high volatility and low viscosity were likely the major contributors to the large post-derailment fireball and pool fire.
4.7
The occurrence crude oil contained concentrations of BTEX that were comparable to typical values reported for crude oils. This explains why concentrations of benzene and other VOCs well above exposure limits were detected at the derailment site.
5.0 Figures
Figure 1 : Photograph showing 2 representative occurrence crude oil samples (NATX310406-C-BOT and NATX310406-C-TOP)
Figure 2 : Atmospheric distillation plots (ASTM D86) for 9 crude oil samples taken from the occurrence train MMA-002
Figure 3 : Atmospheric distillation plots (ASTM D86) for 2 crude oil samples taken from the unit train MMA-874 located at Farnham, Quebec
Figure 4 : Boiling point distribution (ASTM D7169)for 4 crude oil samples taken from the occurrence train MMA-002
Figure 5 : Event tree for release of crude oil from derailed tank cars Footnote 63
6.0 Appendices
Appendix A: Analytical reports provided by external laboratories
- Analytical reports provided by external laboratories (available in PDF only)
This lab report is part of the Transportation Safety Board of Canada's investigation report R13D0054.